Hope for Alzheimer’s. Big news on the horizon for folks in Britain! They might soon get their hands on groundbreaking drugs that could finally slow down Alzheimer’s. These meds, like lecanemab, got the thumbs-up in the US and Japan. And yup, treatments there are already underway. Another drug, donanemab, is gearing up to follow suit. Next year, the UK might greenlight both of these for its people.
A Ray of Hope
It’s a glimmer of hope in the fight against dementia. In the UK alone, a million folks battle this condition, and by 2040, that number might soar to 1.7 million. Last year, 66,000 lives were claimed by dementia in England and Wales. Shockingly, it’s now the top cause of death in Britain, with Alzheimer’s making up two-thirds of cases.
Until now, doctors could only dish out meds to manage symptoms. But now, for the first time ever, these new drugs aim to tackle the root cause of Alzheimer’s, sparking a wave of cautious optimism.
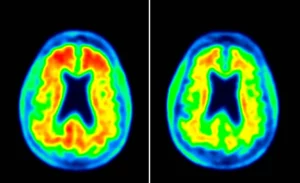
A Step Forward
David Thomas from Alzheimer’s Research UK chimes in. He says these drugs only slow the disease by six months to a year, and they’re best for folks in the early stages. So, they’re not miracle cures, but they’re a start. After decades of research, these meds are the first to directly improve patients’ lives. That’s something to get excited about—it’s a sign that we might finally be heading in the right direction.
Neurologist Cath Mummery from the Dementia Research Centre at University College London echoes the sentiment. It’s been a tough journey, but finally, there’s a glimmer of positivity.
The Amyloid Mystery
Alzheimer’s kicks off with a protein buildup called amyloid in the brain. But here’s the catch: symptoms might not show up until way later. Scientists have been at it for over 20 years, trying to stop amyloid from forming those plaques. Enter lecanemab from Eisai in Japan and donanemab from Eli Lilly in the US. These are the first drugs to slow down the plaque-making—but they don’t stop it altogether.
The Road Ahead
Both drugs are up for UK approval next year. First, the Medicines & Healthcare products Regulatory Agency (MHRA) checks if they’re safe and effective. Then, the National Institute for Health and Care Excellence (Nice) figures out if they’re worth the cost.
But here’s the hitch—they’re pricey. Lecanemab sets you back about $25,000 a year and needs regular IV infusions. Finding space and time for treatments is a hurdle for healthcare services.
Diagnosis Dilemma
Diagnosing dementia is no cakewalk. Most cases start with GPs and then referrals to memory clinics, but the wait times are painfully long—up to two years on average.
Plus, diagnosing Alzheimer’s and other types of dementia involves tests, scans, and often, a long wait for a confirmed diagnosis. About a third of cases are never diagnosed, and that means no access to treatments, including the new drugs.
Personal Stories
Eleanor Mackenzie-Smith’s dad struggled with young-onset Alzheimer’s. It took over a decade and multiple tests before they got a final diagnosis. Waiting and not knowing what’s happening can be heart-wrenching.
Graeme Armstrong’s wife faced a misdiagnosis that delayed getting the right treatment. Getting an accurate diagnosis earlier could have made a world of difference.
A Push for Early Detection
Doctors are eyeing blood tests to quickly spot the disease. But that’s still a few years away. In the meantime, the NHS needs a revamp to diagnose dementia earlier. Catching it in its tracks is key.
Future Hopes
Scientists are optimistic about breakthroughs that could target dementia more effectively, but they’re years down the line. For now, it’s about improving diagnosis and treatment to tackle this brain challenge head-on.
It’s a step forward, but there’s still a long road ahead in the battle against dementia.